
IN THIS ISSUE
New International Certication Eligibility
Requirements
2014 ASCP
i
Satisfaction Survey
IFBLS 31st World Congress
of Biomedical Laboratory Science,
Taipei, Taiwan
14
11
6
www.ascp.org/international
Volume 8 Issue 4 December 2014

2
International Certication Report
International Liaison’s
Message
Editor:
Patricia A. Tanabe, MPA, MLS(ASCP)
CM
Managing Editor and
Production Coordinator:
Joseph Brendan Baker, MS
Editorial Advisor:
Jennifer Young, CT(ASCP)
CM
Graphics and Layout:
Tiffany Cruz-Sugar
International Liaison:
Kathleen Finnegan, MS, MT(ASCP)SH
CM
ASCP
i
Collaborating Societies
CTMP (Colegio Tecnólogo Médico
del Perú)
CONALAC (Colegio de Laboratoristas
Clínicos de Panama)
HKIMLS (Hong Kong Institute of Medical
Laboratory Sciences, Ltd.)
HKSMDS (Hong Kong Society for Molecular
Diagnostic Sciences, Ltd.)
KAMT (Korean Association of Medical
Technologists)
PAMET (Philippine Association of
Medical Technologists, Inc.)
PETIE (Pan-Hellenic Association of
Medical Technologists)
SBPC/ML (Sociedade Brasileira de Patologia
Clínica/Medicina Laboratorial)
SOTEMELAB (Sociedad de Tecnólogos Medicos
en Laboratorio Clínico)
TECMED (Colegio de Tecnólogos Médicos
de Chile)
Regional Representatives
Brijesh Kohli, India, Africa,
and the Middle East
Cristina González del Riego,
Latin America and the Caribbean
Dionysis Vourtsis, Europe
ASCP Board of Certication
Editorial Committee
Scott E. Aikey, MLS(ASCP)CMDLM
CM
- Chair
Susan M. Harrington, PhD, MLS(ASCP)
CM
Perthena A. Latchaw, MS, MLS(ASCP)
CM
Susan E. Morris, MPH, MLS(ASCP)
CM
Lena Spencer, MA, HTL(ASCP)
CM
QIHC
The October 2014 ASCP Annual Meeting “Beyond the Lab” in Tampa, Florida, was an exciting
time for the ASCP BOC international team.
The Annual Meeting, which took place Oct. 8–10 at the Tampa Convention Center, featured ASCP’s
own Dr. E. Blair Holladay, CEO as keynote speaker. The meeting also included numerous important
motions by the BOC Board of Governors (BOG). The BOG approved a number of new eligibility
routes for international applicants. The following international certications will be available Jan.
1, 2015: Technologist and Specialist in Chemistry, Technologist and Specialist in Microbiology,
Technologist in Cytogenetics, and Histotechnologist. The following international certications
are expected to be available in April 2015: Technologist and Specialist in Blood Banking,
Cytotechnologist, Specialist in Cytotechnology, and Histotechnician :(see page 6 for further details).
Additionally, ASCP BOC participated in two large medical laboratory events in Brazil and Taiwan.
In Rio de Janeiro, Brazil, from Sep. 9-12, Jennifer Young, CT(ASCP)
CM
and Latin America
Program Coordinator, Cristina Gonzalez del Riego, promoted ASCP
i
at the Brazilian Society of
Clinical Pathology and Laboratory Medicine (Sociedade Brasileira de Patologia Clínica Medicina
Laboratorial (SBPC/ML—see page 15). And, from Oct. 3-5, Joseph Baker, MS, Manager,
International Certication, ASCP Board of Certication, presented ASCP
i
international certication
to students and professionals at the International Federation of Biomedical Laboratory Scientists
(IFBLS) biennial congress in Taipei, Taiwan (see page 14).
This month also marks my departure as International Liaison. My time as International Liaison has
been a wonderful and rewarding experience which I’m happy to pass on to the new International
Liaison, Kathleen Finnegan, MS, MT(ASCP)SH
CM
. Ms. Finnegan has been a member of the BOG for
the past two years and currently sits on the Policy and Procedure Committee. She holds the title of
Clinical Associate Professor, Department of Clinical Laboratory Sciences, at Stony Brook University
of New York. She received her bachelor’s degree from the State University of New York at Stony
Brook in Clinical Laboratory Sciences and her master’s degree from C.W. Post in Medical Biology.
Her many years of clinical experience were at a small community hospital. She has 20 years of
teaching in the disciplines of hematology, coagulation, and phlebotomy and is currently chair of
the Clinical Laboratory Sciences program and Program Director of the Phlebotomy Training
Program at Stony Brook.
Truly, there are a myriad of wonderful new opportunities available around the world due to the hard
work of the ASCP BOC International team. Please enjoy reading all about it in this latest edition of
the International Certication Report.
Rae Rader MPA, PA(ASCP)
CM
,
Chair, Nominating Committee,
ASCP BOC Board of Governors
Kathleen Finnegan, MS, MT(ASCP) SH
CM
,
International Liaison, International
Consortium for ASCP
i

3
Volume 8 Issue 4 December 2014
International Certication
Ambassador Program
Advisory Boards
Australia
Tony Badrick
Caribbean
Marcia Dean, Jamaica
Uselencia Esajas, Suriname
Clio Innes, Bermuda
Harry Narine, Trinidad
Colin Roach, Guyana
Colombia
Alba Cecilia Garzón
Adriana Martínez
Diana Patiño
Andreas Rothstein
Claudia Cecilia Cardozo Romero
Ecuador
Henry Alvarez
Juan Antonio Cabrera Chacón
Lucía Ulloa
Susana Valladares
Egypt
Hassan Azzazy
Moamena Kamel
Samuel Levin
Hend El Sherbini
Magdy El Ekiaby
El Salvador
Ana Josefa Carranza de Ulloa
Ethiopia
Tedla Mindaye
Greece
Dionysis Vourtsis
Nikolaos Bournousouzis
Petros Papalexis
Anastasios Kriebardis
E Pavlou
Ioannis Zannopoulos
Ioannis Karvounis
Petros Karkalouses
Hong Kong
Chris Wong
Hermia Lam
Gina Leung
Alex Leung
Marianne Leung
Bosco Yau
India
Deepak Kumar Mishra
Amar Nath Sinha
Arun Deshmukh
Pankaj Kaul
Manindra Nath Chaudhurui
Surya Dev
Dhananjay Kumar
Jignesh Dave
Jamaica
Marcia Dean
Sonia Richards-Malcolm
Meredith Williams
Janice Wissart
Warren Williams
Sharon Thompson Kiddo
Paul Gyles
Japan
Hideo Sakamoto
Kunihiro Mimura
Hiromitsu Yokota
Jordan
Maher Sughayer
Salah Bohisi
Salwa Amarin
Randa Al Ahmed
Aktham Haddadin
Nashat Dahabra
Said Ismail
Ismail Matalka
Korea
Kyung Jin Cho
Tae Un Kim
Young Kwon Kim
Moon Jung Shim
Hyo Chan Kang
Jang Ho Lee
Kwang Hyun Ryu
Man-Gil Yang
Chun Hee Kim
Kuwait
Mariam Al Haj Ali
Mohammad Al-Jafar
Reem Ameen
Fatma Al-Yatama
Morocco
Mohamed Abdou
Nigeria
Eugene I. Ikeh
Jelpe Tapdiyel
Lawrena C. Okoro
McPaul I.Jå. Okoye
Pakistan
Usman Waheed
Hasan Abbas Zaheer
Haroon Khan
Muhammad Sarfraz Gondal
Muhammad Asim Ansari
Muhammad Saboor
Panama
Gloriela de Pinzón
Nora Ortiz de Moreno
Boris de León
Nelson Cedeño
Peru
Silvia Flores
Segundo León
Fernando Palacios
José Antonio Paredes
Carlos Penalillo
Patricia Quintana
Cecilia Padilla Salas
Philippines
Agnes B. Medenilla
Marian M. Tantingco
Erlinda C. Pijuan
Soledad L. Bautista
Nini F. Lim
Leila M. Florento
Luella Vertucio
Mark Yulores
Lourdes Gatbonton
Qatar
Hassan Aziz
Saudi Arabia
Abdullah Al-Angery
Hanaa Salem Bameeh
Victoria Rani Leo
Singapore
Ong Siew Kim
Susan Lim Tsui Tsui
Woo Wee Hong
Koh Tse Yuen
Joel Lee
Alvin Poh Lye Hin
Saroj Waikar
Sudan
Faisal Ibrahim
Abdel Mahmoud
Albadri Makki
Alfatih Aljafari
Naser Mohamed
Elwaleed Ibrahim
Alneil Hamza
Taiwan
Wen-Shyang Hsieh
Tze-Kiong Er
Yih-Hsin Chang
Kuo-Chien Tsao
Shu-Chu Shiesh
Kan-Jen Tsai
Shih-Yen Lo
Shiao-Ping Huang
Ya-Hui Chuang
Kuo-Ching Liu
Yung-Feng Lin
Thailand
Rachana Santiyanont
Palanee Ammaranond
Sirirat Tunsakul
Pornsuri Pongsuchart
Trinidad
Harry Narine
Uganda
Ali Elbireer
U. A. E.
Rana Nabulsi
Ahmad Sultan
Samir Awadallah
Mouza AlSharhan
Sumedha Sahni
Soria Sari
Hala Ibrahaim Farah
Abdulrahman
Issam Mayhoub
Suhail Al-Salam
Mohammad Jamali
Kathleen Meehan

4
International Certication Report
Congratulations to Rana Nabulsi, PhD, MSc, CPHQ on her appointment to
Chair the U.A.E. Advisory Board!
KingMed afliated Guangzhou Medical University in China has been reviewed and determined
acceptable in meeting the eligibility requirements of International Medical Technologist MT(ASCP
i
)
certication examination under Route 1.
Labs4Life: Congratulations to the three rst place winners of the 2014 Labs4Life Photo Contest.
See all the submitted photos: www.labsarevital.com/community/photos
The Board of Certication (BOC) Board of Governors Annual Meeting Updates
see pages 6-9 for eligibility requirements:
The Board of Governors approved new international certications.
The following international certications will be available Jan. 1, 2015:
• International Technologist in Chemistry, C(ASCP
i
)
• International Specialist in Chemistry SC(ASCP
i
)
• International Technologist in Cytogenetics CG(ASCP
i
)
• International Histotechnologist, HTL(ASCP
i
)
• International Technologist in Microbiology, M(ASCP
i
)
• International Specialist in Microbiology, SM(ASCP
i
)
The following international certications are expected to be available in April 2015:
• International Histotechnician, HT(ASCP
i
)
• International Technologist in Blood Banking, BB(ASCP
i
)
• International Specialist in Blood Banking, SBB(ASCP
i
)
• International Cytotechnologist, CT(ASCP
i
)
• International Specialist in Cytotechnology, SCT(ASCP
i
)
The current title, “Certication Maintenance Program”, will be changed to
“Credential Maintenance Program” to incorporate both certication and qualication maintenance.
Change in Credential Name for International Medical Technologist—Effective Jan. 1, 2015 the International Medical
Technologist, MT(ASCP
i
) credential will ofcially transition into the International Medical Laboratory Scientist,
MLS(ASCP
i
) credential. The ASCP BOC is transitioning the MT(ASCP
i
) credential to MLS(ASCP
i
) in order to more
closely align it with its U.S. counterpart. All individuals certied beginning Jan. 1, 2015, will be awarded the title
MLS(ASCP
i
). Individuals certied MT(ASCP
i
) prior to Jan. 1, 2015, will transition to MLS(ASCP
i
) upon completion
of the Credential Maintenance Program (CMP) after Jan. 1, 2015. Individuals certied before January 2012 who
are not required to participate in the CMP program and who choose not to do so on a voluntary basis will retain
their MT(ASCP
i
) credential.
January 2015 International Procedures for Examination and Certication—The newest certication
categories available are included in this revised booklet, available online after Jan. 1, 2015
International Addresses—Students living outside of the United States and Canada should be sure
to include their complete addresses on the application form. It is important to indicate the full
street address, city, province/state, and name of the country. Although communication during
the application process is handled by email, the ofcial certicate must be delivered by U.S.
mail. It is important that the address be as complete as possible and that any address changes
are sent directly to [email protected].
ASCP Middle East Meeting—The BOC will sponsor an open forum on ASCP
i
at the ASCP
hosted education event in Abu Dhabi UAE - Dec. 11-12, 2014
ASCP at Arab Health 2015—An ASCP representative will be on site ready to answer
your questions at booth RA32 during the 2015 Medlab Congress at Arab Health, Dubai,
U.A.E., Jan. 26-29, 2015. Additional information on Arab Health and
Medlab is available at: www.medlabme.com
AnnouncementS
Conference Secretariat: MCI Middle East – Tel: +971 4 311 6300, Fax: +971 4 311 6301, Email: [email protected]
Stay Connected:
Conference Topics
Who should attend?
Important Dates
· Leadership Role of Pathology in a Patient-Centered Era
· Implementing Laboratory Management in International Settings
· Counting the Cost of Quality in Medical Laboratories in
Resources Limited Environment
· Putting Pathology and Laboratory Medicine in the Driver’s
Seat for Tomorrow’s Healthcare
· Essential Knowledge and Skills for the Laboratory Manager
· Improving Quality, Efficiency, and Turnaround Time in the
Clinical Laboratory
· Risk Management Approach to Quality Control
· Next Generation Sequencing in Myeloid Malignancies
· In the Eye of the Storm: Role of Molecular Diagnostics
Laboratory in an Outbreak
· Pathologists
· Laboratory professionals
· General practitioners
· Infection control practitioners
· Residents and students
· Laboratory managers and
directors
· Nurses
Conference Dates: 11 - 12 December 2014
Early Bird Registration Opens: 8 October 2014
Online Registration Closes: 4 December 2014
MIDDLEEAST2014
Abu Dhabi - United Arab Emirates
11 - 12 December, 2014
Save the
Dates!
11 - 12
December
2014

5
Volume 8 Issue 4 December 2014
ASCP 2014 Tampa, Florida: “Beyond the Lab”
ASCP 2014 Tampa, Oct. 8–10, at the Tampa Convention Center,
convened the foremost experts and peers in pathology and
laboratory medicine who shared their latest discoveries and
insights on how the medical laboratory profession can improve
patient centered care around the globe and prepare for the future
in this evolving healthcare climate. Over 150 education sessions
were delivered alongside hundreds of scientic research and
medical laboratory management abstracts highlighting new
technologies, techniques, advances in clinical and anatomical
pathology, and more.
This year’s meeting featured ASCP’s own Dr. E. Blair Holladay, CEO
as keynote speaker—discussing the future of healthcare—as well
as self-proclaimed medical futurist Bertalan Mesko, MD, PhD who
challenged attendees to embrace technology and think outside
the box when it comes to the practice of pathology in the future.
Special guest speaker Barbara Pierce Bush discussed her role in
global health as Co-Founder and President of Global Health Corps
and joined an inspirational panel discussion on women in
Leadership and Mentoring. Special guest country music singer
Wade Hayes led the Grand Opening General Session, sharing his
personal experience with surviving stage IV colon cancer and
expressing his thanks to all those in laboratory medicine.
Mr. Hayes debuted his latest song, “Go Live Your Life,”
highlighting the challenge given to him by his doctor when
all the tests showed no cancer present.
Once again, the ASCP BOC was pleased to welcome international
guests and advisory board members; some attending for the rst
time. During the 2014 meeting advisory board members and
international guests participated in an international breakfast
meeting where they could come together to network, meet ASCP
and BOC staff and volunteer leadership, and learn about how to
connect with the society using social media. The group learned
about opportunities available through Facebook, LinkedIn, and
discussed how countries like China could still participate in ASCP’s
social programs through forming their own groups on ASCP’s
OneLab and referencing established BOC boards.
Many of the international advisory board members and guests also
presented during ASCP 2014: “Beyond the Lab.” Ali Elbireer, PhD,
MBA, MT(ASCP), Chair of the Uganda Advisory Board, presented
“Counting the Cost: Cost of Quality in Medical Laboratories in
Resources Limited Environment.” Yih Hsin Chang, PhD, MB(ASCP
i
)
of the Taiwan Advisory Board presented a poster abstract titled
“Association of CTLA-4 Gene Polymorphism and Type 2 Diabetes
Mellitus” and Changshun Yu and Jingwen Zhang, MT(ASCP
i
)
CM
presented research from KingMed in China with a poster presentation.
This year’s annual meeting provided opportunities for Advisory
Board members to connect with staff in one to one meetings to
answer specic questions on application processing and provided
a platform where they could share their ideas on how to best
introduce certications in new markets.
According to Ali Elbireer attending the ASCP Annual meeting is
a very worthwhile endeavor.
“I do make an effort to ensure I continue participating in the
annual ASCP meeting due the great professional and leadership
interactions that are vital in my role as a Laboratory Administrative
Director for an accredited laboratory in a resources limited setting,”
said Dr. Elbireer.
Adding that “as resources for our profession are dwindling world
-
wide and the demand for delivering quality diagnostics at lower
cost to our populations is rising; being an active member of the
ASCP community gives me a personal sense of pride and a venue
to help impact the quality of healthcare delivery to the global
healthcare community.”
Honored International Attendees and
Advisory Board Members at the
International Breakfast Meeting
Changshun Yu—China
Jingwen Zhang, MT(ASCP
i
)
CM
—China
Dr. Henry Alvarez—Ecuador
Chris Lei Po Wong, PhD, FASCP, MB(ASCP
i
)—Hong Kong
Solon Kidane—Mozambique
Lic. TM Silvia Flores Toledo—Peru
Jose C. Jara Aguirre, Bsc, MD—Peru
Yih-Hsin Chang, PhD, MB(ASCP
i
)—Taiwan
Ali Elbireer, PhD, MBA, MT(ASCP)—Uganda
International advisory board members and guests at ASCP 2014: “Beyond the Lab” in Tampa, Florida.

6
International Certication Report
New International Certication Eligibility Requirements
Detailed information on the application process and documentation
required to verify eligibility is available online and in the International
Procedures for Examination and Certication booklet available at
www.ascp.org/international
Technologist in Chemistry, C(ASCP
i
)
Eligibility Routes
ROUTE 1:
MT(ASCP
i
) certication, AND a baccalaureate degree
from an accredited/approved* educational institution; OR
ROUTE 2:
Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
one year acceptable clinical laboratory experience in chemistry
in an accredited/approved** laboratory facility; OR
ROUTE 3:
Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
successful completion of a Chemistry training program*; OR
ROUTE 4:
Graduate level degree (Master’s, Doctorate) in chemistry,
or an appropriately related eld, from an accredited/approved*
educational institution, AND six months acceptable clinical
laboratory experience in chemistry in an accredited/approved**
laboratory facility; OR
ROUTE 5:
Baccalaureate degree in medical laboratory sciences***
from an accredited/approved* educational institution, AND
successful completion of a Medical Laboratory training program*.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved by
an International Advisory Board appointed by the ASCP Board of
Certication, or eligibility will be determined by transcript evaluation.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
***Degrees/Diplomas in Medical Laboratory Sciences include Medical
Laboratory Science, Medical Technology, Clinical Laboratory Science,
and Biomedical Laboratory Science.
Specialist in Chemistry, SC(ASCP
i
)
Eligibility Routes
ROUTE 1:
MT(ASCP
i
) certication or C(ASCP
i
) certication, AND
a baccalaureate degree from an accredited/approved* educational
institution, AND three years of acceptable clinical laboratory
experience in chemistry in an accredited/approved** laboratory
facility. These three years of experience must be acquired post
baccalaureate degree; OR
ROUTE 2:
Graduate level degree (Master’s, Doctorate) in chemistry,
biology, immunology, microbiology, medical laboratory sciences***,
or an appropriately related eld, from an accredited/approved*
educational institution, AND three years acceptable clinical
laboratory experience in chemistry in an accredited/approved**
laboratory facility. These three years of experience must be
acquired post baccalaureate degree; OR
ROUTE 3:
Doctorate in chemistry, biology, immunology, microbiology,
medical laboratory sciences***, or an appropriately related eld,
from an accredited/approved* educational institution, AND two years
of acceptable clinical laboratory experience in chemistry in an
accredited/approved** laboratory facility. These two years of
experience must be acquired post baccalaureate degree; OR
ROUTE 4:
MT(ASCP
i
) certication, AND a doctorate in chemistry,
biology, immunology, microbiology, medical laboratory sciences***,
or an appropriately related eld, from an accredited/approved*
educational institution, AND two years laboratory experience in
chemistry (e.g., clinical, research) in an accredited/approved**
laboratory facility.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved by
an International Advisory Board appointed by the ASCP Board of
Certication, or eligibility will be determined by transcript evaluation.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
***Degrees/Diplomas in Medical Laboratory Sciences include Medical
Laboratory Science, Medical Technology, Clinical Laboratory Science,
and Biomedical Laboratory Science.
Technologist in Microbiology, M(ASCP
i
)
Eligibility Routes
ROUTE 1:
MT(ASCP
i
) certication, AND a baccalaureate degree
from an accredited/approved* educational institution; OR
ROUTE 2:
Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND one
year acceptable clinical laboratory experience in microbiology in an
accredited/approved** laboratory facility; OR
ROUTE 3:
Baccalaureate degree from an accredited/approved*
educational institution in biological science, chemistry or medical
sciences, AND successful completion of a Microbiology program; OR
ROUTE 4:
Graduate level degree (Master’s, Doctorate) in
microbiology, or an appropriately related eld, from an accredited
/approved* educational institution, AND six months acceptable
clinical laboratory experience in Microbiology in an accredited
Online Applications – Available Dec. 1, 2014
First Examination Administration – Jan. 1, 2015

7
Volume 8 Issue 4 December 2014
/approved** laboratory facility; OR
ROUTE 5:
Baccalaureate degree in medical laboratory science***
from an accredited/approved* educational institution, AND successful
completion of a Medical Laboratory training program*.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board of
Certication, or eligibility will be determined by transcript evaluation.
The baccalaureate degree must be equivalent to a U.S. baccalaureate
degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
***Degrees/Diplomas in Medical Laboratory Science include Medical
Laboratory Science, Medical Technology, Clinical Laboratory Science,
and Biomedical Laboratory Science.
Specialist in Microbiology, SM(ASCP
i
)
Eligibility Routes
ROUTE 1:
MT(ASCP
i
) certication or M(ASCP
i
) certication, AND
a baccalaureate degree from an accredited/approved* educational
institution, AND three years of acceptable clinical laboratory
experience in microbiology in an accredited/approved**
laboratory facility. These three years of experience must
be acquired post baccalaureate degree; OR
ROUTE 2:
Graduate level degree (Master’s, Doctorate) in chemistry,
biology, immunology, microbiology, clinical laboratory sciences, or
an appropriately related eld, from an accredited/approved*
educational institution, AND three years acceptable clinical
laboratory experience in microbiology in an accredited/approved**
laboratory facility. These three years of experience must be
acquired post baccalaureate degree; OR
ROUTE 3:
MT(ASCP
i
) certication, AND a doctorate in chemistry,
biology, immunology, microbiology, clinical laboratory sciences, or an
appropriately related eld, from an accredited/approved* educational
institution, AND two years laboratory experience in microbiology (e.g.,
clinical, research) in an accredited/approved** laboratory facility.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to a
U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Technologist in Cytogenetics, CG(ASCP
i
)
Eligibility Routes
ROUTE 1:
Baccalaureate degree from an accredited/approved*
educational institution, AND successful completion of an
accredited/approved* Cytogenetics education program; OR
ROUTE 2:
Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
one year acceptable work experience in an accredited/approved**
cytogenetics*** laboratory or an accredited/approved** laboratory; OR
ROUTE 3:
Graduate level degree (Master’s or Doctorate) in genetics
or molecular biology from an accredited/approved* educational
institution, AND nine months acceptable work experience in an
accredited/approved** cytogenetics*** laboratory or an
accredited/approved** laboratory.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
***A cytogenetics laboratory is dened as one capable of providing
individuals with knowledge and practical experience in cytogenetics,
including culturing, harvesting, staining, microscopic chromosome
analysis and molecular cytogenetics.
Histotechnologist, HTL(ASCP
i
)
Eligibility Routes
ROUTE 1:
Baccalaureate degree from an accredited/approved*
educational institution, AND successful completion of an
accredited /approved* Histotechnician or Histotechnology
program; OR
ROUTE 2:
Baccalaureate degree from an accredited/approved*
educational institution, AND one year acceptable experience in a
histopathology (clinical, veterinary, industry, or research) laboratory
in an accredited/approved** laboratory facility.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved

8
International Certication Report
by an International Advisory Board appointed by the ASCP Board of
Certication.
Technologist in Blood Banking,
BB(ASCP
i
) Eligibility Routes
ROUTE 1: MT(ASCP
i
) certication, AND a baccalaureate degree
from an accredited/approved* educational institution; OR
ROUTE 2: Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
one year acceptable clinical laboratory experience in Blood
Banking (Immunohematology) in an accredited/approved**
laboratory facility; OR
ROUTE 3: Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
successful completion of a Blood Banking (Immunohematology)
program; OR
ROUTE 4: Graduate level degree (Master’s, Doctorate) in chemistry,
biology, immunology, immunohematology, microbiology, clinical
laboratory sciences or an appropriately related eld, from an
accredited/approved* educational institution, AND six months
acceptable clinical laboratory experience in Blood Banking
(Immunohematology) in an accredited/approved**
laboratory facility; OR
ROUTE 5: Baccalaureate degree in medical laboratory science***
from an accredited/approved* educational institution, AND
successful completion of a Medical Laboratory training program*.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
***Degrees/Diplomas in Medical Laboratory Science include Medical
Laboratory Science, Medical Technology, Clinical Laboratory Science,
and Biomedical Laboratory Science.
Specialist in Blood Banking, SBB(ASCP
i
)
Eligibility Routes
ROUTE 1: Baccalaureate degree from an accredited/approved*
educational institution in biological science or chemistry, AND
successful completion of a minimum one-year post baccalaureate
Blood Bank (Immunohematology) program*; OR
ROUTE 2: MT(ASCP
i
) or BB(ASCP
i
) certication, AND a
baccalaureate degree from an accredited/approved* educational
institution, AND three years of acceptable clinical laboratory
experience in Blood Banking (Immunohematology) in an
accredited/approved** laboratory facility. These three years of
experience must be acquired post baccalaureate degree; OR
ROUTE 3: Graduate level degree (Master’s, Doctorate) in chemistry,
biology, immunology, immunohematology, microbiology, clinical
laboratory sciences, or an appropriately related eld, from an
accredited/approved* educational institution, AND three years
of acceptable clinical laboratory experience in blood banking
(immunohematology) in an accredited/approved** laboratory
facility. These three years of experience must be acquired post
baccalaureate degree.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Cytotechnologist, CT(ASCP
i
)
Eligibility Routes
(This certication is not valid for employment in the
United States. By law, individuals interested in working
in Cytotechnology in the United States must complete a
CAAHEP accredited Cytotechnology program.)
ROUTE 1: Baccalaureate degree or higher from an accredited
/approved* educational institution, AND successful completion
of an accredited/approved* Cytotechnology program; OR
ROUTE 2: Baccalaureate degree or higher from an accredited
/approved* educational institution, AND three (3) years of
experience in gynecological and non-gynecological cytology, ne
needle aspiration (FNA), and laboratory operations immediately
prior to application in an accredited/approved** laboratory facility,
AND successful completion of the national examination in countries
where a national registry for Cytotechnology exists; OR
ROUTE 3: Baccalaureate degree or higher from an accredited
/approved* educational institution, AND CT(IAC) certication; OR
ROUTE 4: Baccalaureate degree or higher from an accredited
/approved* educational institution, AND CTgyn(ASCP
i
) certication,
AND two (2) years of experience in non-gynecological cytology, ne
needle aspiration (FNA), and laboratory operations in an accredited/
approved** laboratory facility.
Online Applications – Available March 1, 2015
First Examination Administration – April 1, 2015

9
Volume 8 Issue 4 December 2014
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Specialist in Cytotechnology, SCT(ASCP
i
)
Eligibility Routes
(This certication is not valid for employment in the
United States. By law, individuals interested in working
in Cytotechnology in the United States must complete a
CAAHEP accredited Cytotechnology program.)
ROUTE 1: CT(ASCP
i
) certication, AND a baccalaureate degree or
higher from an accredited/approved* educational institution, AND
three (3) years of acceptable experience in cytology in an accredited/
approved** laboratory facility. These three years of experience must
be obtained following CT(ASCP
i
) certication.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation/
approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP
Board of Certication, or eligibility will be determined by
transcript evaluation. The baccalaureate degree must be
equivalent to a U.S. baccalaureate degree.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Histotechnician, HT(ASCP
i
)
Eligibility Routes
ROUTE 1: Successful completion of an accredited/approved*
Histotechnician program; OR
ROUTE 2: Minimum of a two-year diploma from an accredited
/approved* educational institution, AND one year acceptable
experience in a histopathology (clinical, veterinary, industry, or
research) laboratory in an accredited/approved** laboratory facility.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved by
an International Advisory Board appointed by the ASCP Board of
Certication, or eligibility will be determined by transcript evaluation.
**Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Detailed information on the application process and
documentation required to verify eligibility is available
online and in the International Procedures for Examination
and Certication booklet available at www.ascp.org/international
Technologist in Gynecologic
Cytology(ASCP
i
) Eligibility Routes
ROUTE 1: Minimum of a 2 year diploma or equivalent from an
accredited/approved* educational institution, AND successful
completion of an accredited/approved* Cytotechnology program
in which the program of study incorporates theory and practice
in gynecologic cytology; OR
ROUTE 2: Current employment in the eld of cytology, AND one (1)
year of experience in gynecologic cytology immediately prior to
application in an accredited/approved** laboratory facility, AND
successful completion of the national examination in countries
where a national registry for Cytotechnology exists; OR
ROUTE 3: CT(IAC) certication.
*Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board of
Certication, or eligibility will be determined by transcript evaluation.
**Laboratory accredited by JCI, CAP, under ISO 15189 or
authorized by a governing regulatory association or Ministry.
Countries without a prevalent system of accreditation must
have laboratories approved by an International Advisory Board
appointed by the ASCP Board of Certication.
Revised International Certication Eligibility Requirements
Effective Immediately

10
International Certication Report
Medical Laboratory Technician,
MLT(ASCP
i
) Eligibility Routes
ROUTE 1: Minimum of a two-year diploma in Medical Laboratory
Technology* which includes training in blood banking (immunohema-
tology), chemistry, hematology, and microbiology from an accredited/
approved technical institution**; OR
ROUTE 2: Minimum of a two-year diploma or equivalent in any biologi-
cal science or chemistry from an accredited/approved** educational
institution, AND successful completion of a Medical Laboratory
training program**. The training program must include blood banking
(immunohematology), chemistry, hematology, and microbiology; OR
ROUTE 3: Minimum of a two-year diploma or equivalent in any biologi-
cal science or chemistry from an accredited/approved** educational
institution, AND three years experience including all aspects of the
clinical laboratory in an accredited/approved*** laboratory facility.
Experience must include blood banking (immunohematology), chem-
istry, hematology, and microbiology.
*Degrees/Diplomas in Medical Laboratory Technology include Medical
Technology, Medical Laboratory Science, Clinical Laboratory Science,
and Biomedical Laboratory Science.
**Accredited/approved by a governing regulatory association or Min-
istry. Countries without a prevalent system of accreditation/approval
must have programs/educational institutions approved by an Interna-
tional Advisory Board appointed by the ASCP Board of Certication, or
eligibility will be determined by transcript evaluation.
***Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication.
Technologist in Molecular Biology,
MB(ASCP
i
) Eligibility Routes
ROUTE 1: ASCP
i
certied as a technologist (MT, CTgyn), AND
a baccalaureate degree from an accredited/approved**
educational institution; OR
ROUTE 2: Baccalaureate degree from an accredited/approved**
educational institution, AND successful completion of an
accredited /approved** diagnostic molecular science program.
Academic education or molecular science program must include
courses in biological science and chemistry; OR
ROUTE 3: Baccalaureate degree from an accredited/approved**
educational institution, AND successful completion of a minimum
two-year Medical Laboratory program**. The training program must
include blood banking (immunohematology), chemistry, hematology,
and microbiology; OR
ROUTE 4: Baccalaureate degree in any biological science or chemistry
from an accredited/approved** educational institution, AND one year
acceptable experience (clinical, veterinary, industry or research) in a
molecular biology laboratory* in an accredited/approved***
laboratory; OR
ROUTE 5: Graduate level degree (Master’s, Doctorate) in any biologi
-
cal science or chemistry from an accredited/approved** educational
institution, AND six months acceptable experience (clinical, veteri-
nary, industry, or research) in a molecular biology laboratory* in an
accredited/approved*** laboratory.
*A molecular biology laboratory is dened as one capable of providing
individuals with knowledge and practical experience in all aspects of
molecular analysis including, but not limited to, recombinant DNA
technologies, polymerase chain reaction, and hybridization techniques.
**Accredited/approved by a governing regulatory association or
Ministry. Countries without a prevalent system of accreditation
/approval must have programs/educational institutions approved
by an International Advisory Board appointed by the ASCP Board
of Certication, or eligibility will be determined by transcript
evaluation. The baccalaureate degree must be equivalent to
a U.S. Baccalaureate degree.
***Laboratory accredited by JCI, CAP, under ISO 15189 or
authorized by a governing regulatory association or Ministry.
Countries without a prevalent system of accreditation must have
laboratories approved by an International Advisory Board appointed
by the ASCP Board of Certication.
Phlebotomy Technician, PBT(ASCP
i
)
Eligibility Routes
ROUTE 1: High school graduation (or equivalent), AND completion of a
formal phlebotomy program. This program must consist of: classroom
training, including anatomy and physiology of the circulatory system,
specimen collection, specimen processing and handling and labora-
tory operations (e.g. safety, quality control, etc.), AND clinical training
in an approved/accredited laboratory* with a minimum performance
of 50 successful, unaided blood collections, including venipunctures
and skin punctures; OR
ROUTE 2: High school graduation (or equivalent), AND completion of
one year acceptable work experience as a phlebotomy technician in
an approved/accredited laboratory*. This experience must include
venipunctures and skin punctures; OR
ROUTE 3: High school graduation (or equivalent), AND successful
completion of a nursing or other acceptable/accredited biomedical
science education which includes phlebotomy training with a minimum
performance of 50 successful, unaided blood collections, including
venipunctures and skin punctures; OR
ROUTE 4: MT(ASCP
i
) or MLT(ASCP
i
) certication.
Applicants with training in the United States must apply through
United States PBT(ASCP) eligibility routes.
*Laboratory accredited by JCI, CAP, under ISO 15189 or authorized
by a governing regulatory association or Ministry. Countries without
a prevalent system of accreditation must have laboratories approved
by an International Advisory Board appointed by the ASCP Board of
Certication
11
Volume 8 Issue 4 December 2014
“Certication is encouraged in the Philippines because it
validates people’s skills and knowledge in the eld so that
employers have a good idea as to whether you are qualied,
and, although there is a national certifying body in the
Philippines that regulates medical laboratory scientists,
we are still encouraged to advance our professionalism via
the ASCP
i
certication.”
— Ruffa Jamaica C. Dela Cruz, MT(ASCP
i
)
In April and May 2014, Joseph Baker, MS, Manager, International
Certication, ASCP Board of Certication, and Patrick Fisher, MA,
Manager, Evaluation & Statistics, ASCP Board of Certication,
developed a survey instrument to gauge an ASCP
i
certicant’s
sense of fulllment, personally and professionally, with having
earned ASCP
i
certication.
In June, electronic invitations to participate in the survey were sent
to all active ASCP
i
certicants: PBT(ASCP
i
), MB(ASCP
i
), MT(ASCP
i
),
MLT(ASCP
i
), CTgyn(ASCP
i
). The survey remained active through
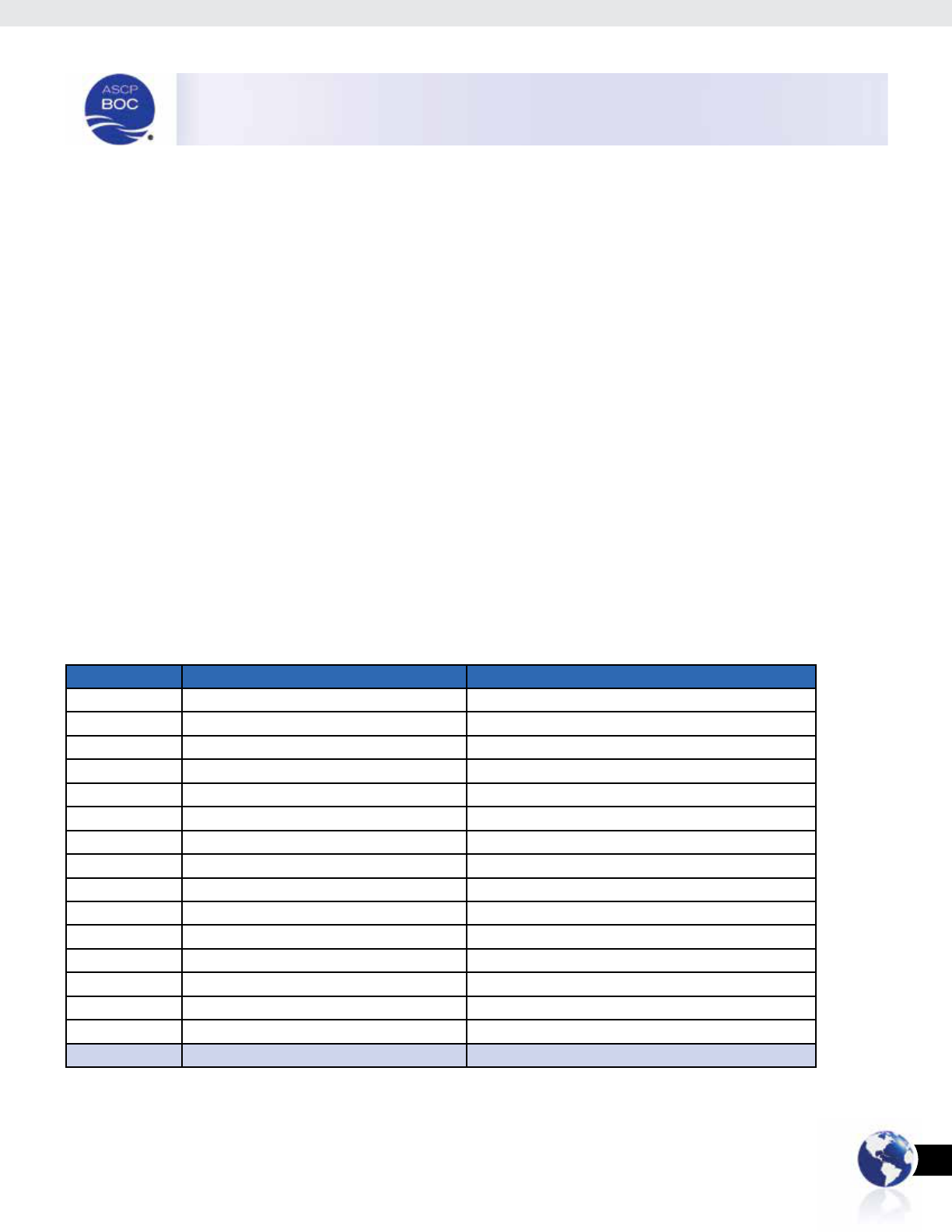
June 30, 2014. In all, 111 individuals from 15 different countries
of education completed the survey (Table 1).
Findings
A total of 112 responses were received, but only 111 were completed.
In terms of the respondent’s country of education, the following top
ve countries provided the majority of survey responses:
Philippines (77 percent), Jamaica (5 percent), Ghana (3 percent),
China (3 percent), and South Korea (2 percent).
In terms of the overall response rate, the survey skews heavily
towards individuals educated in the Philippines (Table 1).
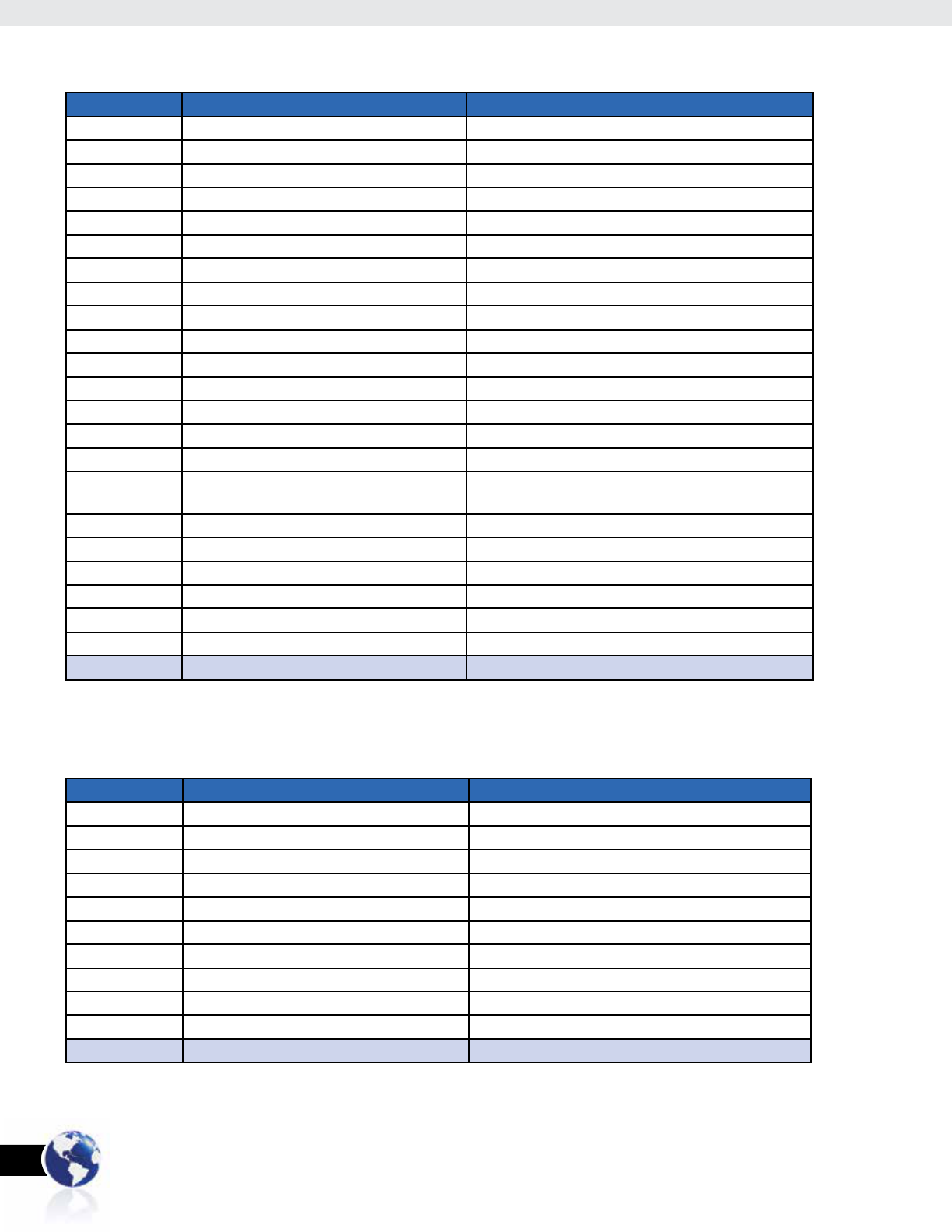
However, when survey participation rate by country of residence is
taken into account, participation by those living in the Philippines
drops to less than 25 percent (Table 2). The top 5 countries, in
terms of response by country of residence are: U.S. (44 percent),
Philippines (22 percent), U.A.E. (6 percent), Saudi Arabia
(4 percent), and Jamaica (4 percent).
This indicates that certicants from the Philippines tend to be the
most uid in terms of relocation to a new country of residence after
receiving their education. Of the 86 respondents who claimed the
Philippines as their country of education, 62 claimed a country
outside of the Philippines as their current residence. Of these 62
Filipinos that currently live outside the Philippines, 63 percent reside
in the U.S., 11 percent reside in the U.A.E., and 8 percent reside
in Saudi Arabia (Table 3). The remaining 18 percent reside in the
following countries: Australia, Bangladesh, Canada, Guam (USA),
Kuwait, Qatar, and Singapore.
ASCP
i
Satisfaction Survey Results
Country # of Respondents % of Total Respondents
Philippines 86 77.4%
Jamaica 6 5.4%
China 3 2.7%
Ghana 3 2.7%
South Korea 2 1.8%
United States *2 1.8%
Burma 1 0.9%
Colombia 1 0.9%
Ethiopia 1 0.9%
Jordan 1 0.9%
Kenya 1 0.9%
Nigeria 1 0.9%
Taiwan 1 0.9%
Uganda 1 0.9%
Venezuela 1 0.9%
TOTAL 111 100.0%
*Respondent error in self-reporting: an international certicant’s eligible education, must be completed
outside of the United States.
Table 1: Respondents by Country of Education
12
International Certication Report
Country # of Respondents % of Total Respondents
United States 39 62.9%
U.A.E. 7 11.2%
Saudi Arabia 5 8.0%
Australia 2 3.2%
Canada 2 3.2%
Guam (USA) 2 3.2%
Kuwait 2 3.2%
Bangladesh 1 1.6%
Qatar 1 1.6%
Singapore 1 1.6%
TOTAL 62 100.0%
Table 3: Filipinos Claiming a Country Outside of the Philippines as Their Current Residence
Country # of Respondents % of Total Respondents
United States 49 44.1%
Philippines 24 21.6%
U.A.E. 7 6.3%
Saudi Arabia 5 4.5%
Jamaica 4 3.6%
Australia 2 1.8%
Canada 2 1.8%
China 2 1.8%
Guam (USA) 2 1.8%
Kuwait 2 1.8%
Bahamas 1 0.9%
Bangladesh 1 0.9%
Belize 1 0.9%
Bermuda 1 0.9%
Burma 1 0.9%
Cayman
Islands
1 0.9%
Jordan 1 0.9%
Qatar 1 0.9%
Singapore 1 0.9%
South Korea 1 0.9%
Taiwan 1 0.9%
Uganda 1 0.9%
TOTAL 111 100.0%
Table 2: Respondents by Country of Residence
13
Volume 8 Issue 4 December 2014
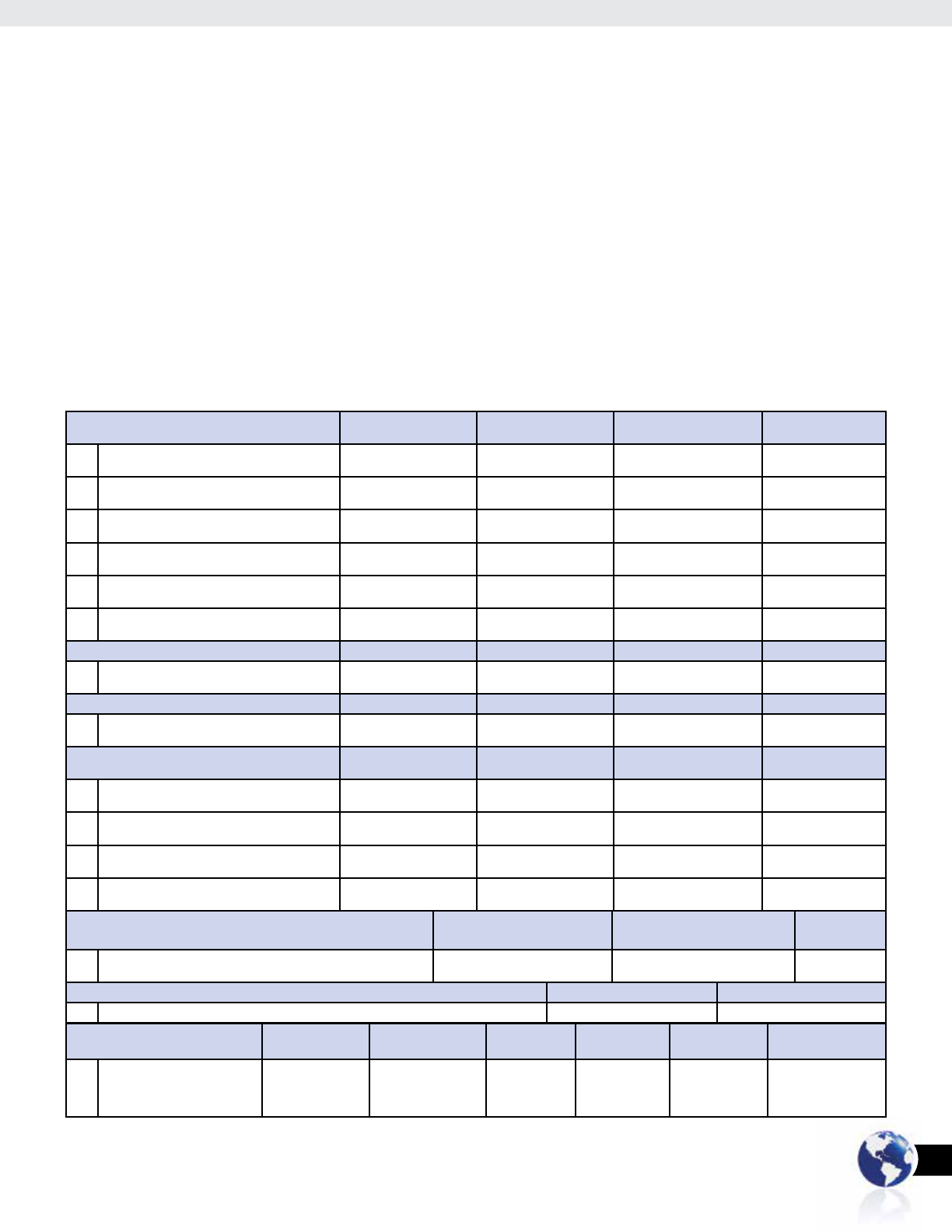
The rst portion of the survey focused on identifying the personal
and professional motivations for why individuals sought certication.
Respondents seeking certication because it was mandated by an
employer accounted for 34 percent of responses (Table 4, Question
1), and 50 percent sought certication because of an employer or
educator recommendation (Table 4, Question 2). Pay and salary
increases as a motivating factor for seeking ASCP
i
certication
accounted for 54 percent of respondents (Table 4, Question 3),
and 68 percent reported they were motivated because they felt
ASCP
i
certication would help them immigrate to another
country (Table 4, Question 4).
The second portion of the survey sought to gauge respondent
satisfaction with taking the examination, and with the personal
and professional benets they have received as a result of attaining
ASCP
i
certication. The majority of respondents (76 percent) found the
ASCP
i
application process easy or very easy (Table 4, Question 7), and
93 percent found ASCP
i
to be useful in their careers (Table 4, Question 8).
A signicant majority of respondents, 96 percent, also reported that
earning the ASCP
i
credential has made them feel more condent (
Table 4,
Question 9),
and 95 percent said it made them feel more professional
(Table 4, Question 10).
In terms of professional benefits, 34 percent of respondents
reported that receiving ASCP
i
certification helped them
receive an increase in pay (Table 4, Question 11), and 29 percent
reported attaining the ASCP
i
credential helped them receive a pro-
motion at work (Table 4, Question 12). In terms of professional de-
velopment, 96 percent reported feeling more confident about their
future in the clinical laboratory field as a result of earning the
ASCP
i
credential (Table 4, Question 15), and another 96 percent
said they took the ASCP
i
examination to validate their knowledge
and skills (Table 4, Question 5). Certificants desiring to use the
ASCP
i
credential for resume or portfolio purposes accounted for 81
percent of respondents (Table 4, Question 6).
All respondents (100 percent) reported they would recommend
ASCP
i
certification to a colleague or friend who is a medical
laboratory scientist, technologist, or technician (Table 4,
Question 13), and 47 percent reported ASCP
i
certification
is encouraged as a part of employment in their region or
country (Table 4, Question 14).
Table 4: 2014 ASCP
i
Satisfaction Survey Results
Personal and Professional Motivation for ASCP
i
Certication
Strongly Agree Agree Disagree Strongly Disagree
1 “I took the ASCP
i
exam because it was mandated
by my employer”
16% 18% 41% 25%
2 “I took the ASCP
i
exam because my
employer/educator recommended I take it.”
25% 25% 31% 19%
3 “I took the ASCP
i
exam because I want a higher
salary/increased pay.”
25% 29% 34% 12%
4 “I took the ASCP
i
exam in order to help me
immigrate to another country.”
44% 24% 24% 8%
5 “I took the ASCP
i
examination to validate my
knowledge and skills.”
61% 35% 4% 0%
6 “I took the ASCP
i
examination for resume
/portfolio purposes.”
28% 53% 14% 5%
Ease of Application Process Very Easy Easy Difcult Very Difcult
7 “I found applying for ASCP
i
certication to be:”
22% 54% 22% 2%
Useful for Career Sucess Useful Mostly Useful Somewhat Useful No Really Useful
8 “Has ASCP
i
certication been useful to you in
your career?”
50% 23% 20% 7%
Personal and Professional Benets of
ASCP
i
Certication
Strongly Agree Agree Disagree Strongly Disagree
9 “Earning the ASCP
i
credential has made me feel
more condent.”
59% 37% 4% 0%
10 “Earning the ASCP
i
credential has made me feel
more professional.”
60% 35% 5% 0%
11 “ASCP
i
certication helped me receive an
increase in pay at work.”
7% 27% 46% 20%
12 “ASCP
i
certication helped me receive a
promotion at work.”
18% 22% 53% 7%
Recommend ASCP
i
? I would Highly Recommend I Would Recommend I Would Not
Recommend
13 “Would you recommend ASCP
i
certication to a colleague or friend who
is a medical laboratory scientist technologist, or technician?”
68% 32% 0%
ASCP
i
in your Region or Country Yes No
14 “Is ASCP
i
certication encouraged as a part of employment in your region or country?” 47% 53%
Future in the Clinical Laboratory Field Extremely Condent A Lot More Condent More Condent Somewhat More
Condent
Only a Little More
Condent
No More Condent
than I was Before
15 “How do you feel about your
future in the clinical laboratory
eld as a result of earning the
ASCP
i
credential?”
26% 39% 21% 7% 3% 4%

14
International Certication Report
Every Two Years, the International Federation of Biomedical
Every Two Years, the International Federation of Biomedical
Laboratory Scientists (IFBLS) sponsors a World Congress of
Biomedical Laboratory Science which is attended by faculty,
administrators, directors, advisors, professionals, students
and others in biomedical laboratory science practice and
education. The 2014 congress took place at the Taiwan
International Convention Center in Taipei from October 3-5.
The IFBLS congress provides a variety of student forums,
educational sessions, exhibits, poster presentations, work-
shops, and networking opportunities, specifically geared
toward medical laboratory science educators, professionals,
and students. The BOC has regularly attended the biennial
IFBLS congress, including: Berlin, Germany 2012, Nairobi,
Kenya 2010, New Delhi, India 2008, and Seoul, South
Korea, 2006.
Joseph Baker, MS, Manager, ASCP BOC International Certication,
presented an introduction to ASCP
i
certication to 120 students
and professionals during a professional development forum at IFBLS
2014. The presentation included background information on ASCP,
the BOC and ASCP
i
, an overview of the application process, why
international certication is important, and the location of the
three Taiwan testing sites.
A number of ASCP BOC Advisory Board members and chairs
were also in attendance at IFBLS 2014 including: Dionysis
Vourtsis, BSc, European Regional Representative and Chair
of the Greece Advisory Board, Hideo Sakamoto, PhD, Chair
of the Japan Advisory Board, Dr. Mangil Yang, President
of KAMT and member of the Korean Advisory Board, Hya
Chan Kang member of the Korean Advisory Board, Manindra
Chaudhuri, MD, Chair of the India Advisory Board, Agnes
Medenilla, MT(ASCP), Chair of the Philippines Advisory
Board, Luella Vertucio member of the Philippines Advisory
Board, Leila Florento, MS, PhD, RMT (PRC), member of the
Philippines Advisory Board, and Alba Marzo, PhD,
[tentative] Chair of the Italy Advisory Board.
IFBLS 31st World Congress of Biomedical Laboratory Science,
Taipei, Taiwan
Join the ASCP
BOC at the Following Upcoming International Events:
ASCP Middle East Conference 2014
Abu Dhabi, UAE—Dec. 11-12, 2014
http://www.ascp.org
2015 Medlab at Arab Health
Dubai, U.A.E.—Jan. 26-29, 2015
http://www.medlabme.com
ASCP Annual Meeting
Long Beach, CA, USA—Oct. 31-Nov. 3, 2015
http://www.ascp.org
(Center) Dionysis Vourtsis, European Regional Representative and
Chair of the Greece Advisory Board, with (left) Chuan-Liang Kao,
former Taiwan Advisory Board Member and Scientic Committee
Chairperson, and (right) Wen C. Tsai, PhD, MT(ASCP), President
TTQAA and Superlab Co., Professor, National Yang-Ming University
Joseph Baker with members of the Philippine Association of Medical
Technologists (PAMET) Board

15
Volume 8 Issue 4 December 2014
The Brazilian Society of Clinical Pathology and Laboratory
Medicine (Sociedade Brasileira de Patologia Clínica
Medicina Laboratorial (SBPC/ML)), is a nonprot organization
founded in 1944 and based in Rio de Janeiro. It is a member-
ship based organization which provides its members, physicians
and specialists, with continuous scientic and technical updates
through events and publications. SBPC/ML’s largest annual
event is the Congresso Brasileiro de Patologia Clínica/Medicina
Laboratorial (CBPCML), a technical-scientic congress for
laboratory professionals.
This annual event acts as a forum for highlighting and
discussing the advances, challenges and accomplishments
occurring in clinical pathology and medical laboratory science.
“The event is already well known within the clinical pathology
sector,” said Wilson Shcolnik, MD, president of the 48th
Congress and Director of Accreditation and Quality SBPC/ML,
in order to highlight the participation of the event’s keynote
speakers, as well as the attendance of representatives from the
ANS (Agência Nacional de Saúde Suplementar/National Health
Agency: National Regulatory Agency for Private Health Insurance
and Plans) and ANVISA (Brazilian Health Surveillance Agency)
in the scientic program.
This year’s congress, held from Sep. 9-12 in Rio de Janeiro,
was attended by approximately 4,500 participants, composed
of medical laboratory professionals and students, clinical
pathologists, speakers and exhibitors. The conference
provides a variety of scientific sessions along with exhibits,
poster presentations, roundtables and other information that
provides networking opportunities specifically geared toward
clinical laboratory professionals and managers.
According Shcolnik, the current congress maintained the
SBPC/ML tradition of focusing on education by offering
continuing education credits for attending the congress,
and by launching publications such as the SBPC/ML book
titled “SBPC/ML Recommendations: Best Practices in
Clinical Microbiology.”
ASCP and BOC co-sponsored an exhibit booth during the congress,
which offered information on ASCP
i
international certication and
continuing education opportunities. Representing ASCP BOC were
Director of International Operations, Jennifer Young, CT(ASCP)
CM
and Latin America Program Coordinator, Cristina Gonzalez del Riego.
Professionals who stopped by the booth mostly inquired about the
local benets in obtaining ASCP BOC international certications as
well as the continuing education products and services available to
members and non-members, and pathology residents.
48th Congress of SBPC/ML, Rio de Janeiro, Brazil
From left to right: Cristina Gonzalez del Riego, ASCP BOC, Latin America Program Coordinator, and
Jennifer Young, ASCP, Director of International Operations

International Certication Report
Congratulations to our newest ASCP
i
International Certificants!
Certied August 27, 2014, through November 7, 2014
International Certication Report
Examinations Available Now:
International Technologist in Hematology, H(ASCP
i
)
International Medical Technologist, MT(ASCP
i
)
Tecnólogo Médico Internacional, MT(ASCP
i
) en español
International Medical Laboratory Technician, MLT(ASCP
i
)
International Technologist in Molecular Biology, MB(ASCP
i
)
International Phlebotomy Technician, PBT(ASCP
i
)
International Technologist in Gynecologic Cytology, CTgyn(ASCP
i
)
Qualications Available Now:
International Qualication in Laboratory Operations, QLO
Qualication in Cytometry, QCYM
Coming Soon:
International Histotechnician, HT(ASCP
i
)
International Cytotechnologist, CT(ASCP
i
)
International Technologist in Chemistry, C(ASCP
i
)
International Technologist in Microbiology, M(ASCP
i
)
International Histotechnologist, HTL(ASCP
i
)
International Blood Banking, BB(ASCP
i
)
International Specialist in Cytotechnology, SCT(ASCP
i
)
International Specialist in Chemistry, SC(ASCP
i
)
International Specialist in Microbiology, SM(ASCP
i
)
International Specialist in Hematology, SH(ASCP
i
)
International Specialist in Blood Banking, SBB(ASCP
i
)
33 W. Monroe St. Ste. 1600
Chicago, IL 60603
U.S.A.
Phone: +1 312.541.4473
Fax: +1 312.541.4845
E-mail: [email protected]
visit our web site at
www.ascp.org/international
International Medical
Technologist, MT(ASCP
i
)
Anastacia Somera
Cristine Mae Castro Pagaragan
Ma Clarita Yonson
Olivia Ongat Awika
Gloria Sacyabon Sagyaman
Mary Lou Estabillo Antonio
Melanie Hernandez Sta Ana
Francis Kyei Bugyei
Yanique Sakita White
Ralph Balicolon Cubangbang
Sinyeon Kim
Krittayaporn Satapanawong
Mariel Rosanne Mozo Lim
Kathleen Denise Naval Oanes
Somin Kim
Engelbert Ferrer Vinoya
Hiwot Kucha Dewele
Jin Seok Kil
Patricia Marie Barrientos Dichoso
Diana Rei Bernardo Legaspi
Seirra Jane Dimaculangan Bugawan I
Soha A Ali
Sulafa S Edress
Jake Camiguing Dorol
Carlo Marty Punzal Manuzon
Ralph Reiner Santos Cunanan
Teodulo Bernardo Jutara Jr
Elizabeth Dulce Faina
Andrew Vittorio Evangelista Dela Pena
Mark Lester Cantal Montabon
Gladys Oamil Fernandez
Roseloraine Norio Sertimo
Ala Adil Badawi
Amal Hassan Mohamed
Leana Andrea Morales Muncal
Amani Ibrahim Ahmed
Erwin Luy Cabrera
May Lanie Canoy Cabalog
Rapunzelle Pagalan Dakoykoy
Regrine Bolando Lagarteja
Debbi Viljoen
Gizette Ann Valencia
Cherry Mae Peredo Trovela
Jennica Rose Marasigan Valentino
Lee Kian Tai
Sohila Elshafee
I Han Wang
Mazelle Vismonte Ferrer
Gladys Cruz Francisco
Ma Cyrell Cinco Arcueno
Bianca Liwag Bigyan
Mara Joy Lithel Bendaña Lorenzo
Shaza Ahmed Marzoug
Joanne Ocampo Vinculado
Hari Jeon
Kathrina Garcia Arriola
Maria Rosana Ocampo France
Tewodros Zerihun Gebre
Shafaf Narangoli
Mi Hee Kong
Venna Supena Ardales
Adnan Yaqoob
Laarni Tiongson Tuason Estrada
Delanee Rose Danna Tabar Pino
Aseil Mutasim Ahmed
Mary Jingle De Guzman Lopez
Richmond Ofe-Brobey Jr
Berlette G Corpuz
Tedla Mindaye Kelecha
Melissa Marie Esperat
Edmund Mawutor Agbi
Hawazen Abshr Elbharey
Melvyn Douglas Pingol Flores
Viola Grace Bumanlag Macaoili
Gina Recla Ocampo
Maria Judy Sadsad Decal
Karel Jayme Guiamano
Michael Salvador Evangelista
Myra Del Rosario Castillo
Jon Jose Calabucal Balintona
Jecyval Garcia Calanno
Surveilla Domingo Sotelo
Jan Marc Conste Punongbayan
Maria Luisa Dicam Tanacio
Anna Victoria Borja Cruz-Symaco
Danielle Mae Chua Rosello
Cecelle Manoza Fillone
Mary Claire Brabante Onde
Gellyn Mae Guanzon Espinosa
Angierica Michelle Catigan Tibayan
Shyra Rina Cabatingan Sale
Irish Mae Ade Cirunay
Gicel Ann Plenos Nudalo
Catherine Grace Santos Adaya
Anne Kathleen Boñon Arcinue
Joomyung Woo
Manuel Resaba Del Rosario
Tanya Kathleen Songcuan Nalapo
Stessy Bovida Caraig
Errol Escueta Coderes
Marie Jasmine Pefanio Sena
Alvin Troy Gonzales Silvano
Claire Anne Wabe Tiu
Alexander Alarde Azana
Thyron Medina Zafra
Julianne Santiago Amador
Regin Clenn Ortiz
Neziela Mae Paete Sabandal
Frank Dioso
Leian Dara Ranario Castro
Cherey Dawn Andaya Saycon
Hyangran Hong
Maria Jhoanna Daban Reantaso
Verlyn Caballes Vergara
Rasha Abdeldaim Alsiddig
Reynelyn Brunie Bayot Anoos
Reneepearl Kim Picana Sales
Aileen Grace Chavez Lim
Laarni Oliva Batin
Nadia Abdelmajeed Madi
Andrew Escano
Noon Zain Hassan
Arlene Carvajal Castillo
Rhea David Villapana
Jeanette N Linaugo
Chrysdale Maghuyop Mallorca
Cornelius Ibe Arce
Yvonne Maxine Wilson
Maria Lota Buaga Versoza
Lora Mae Tecson Lagra
Delia De la Cruz Alfonso
Ella Mae Yap Gupana
Areum Nam
Keith de Josef Begona
April Divedor Painaga
Edwin Michira Kambaga
Shamil Mohamed Anamangadan
Jose Ivan Pasillas
Maria Christen Bautista Pachoco
Jeannie Go Buot
International Technologist
in Molecular Biology, MB(ASCP
i
)
Yu Mi Jeong
Xin Li
Mang Chung Yu
International Medical
Laboratory Technician,
M LT ( A S C P
i
)
Tilahun Muchie Hiwotu
Ravindra Kumar Sukhlal Devre
Muiz Abdelrahman Imam
Su Jin Kang
Khalid Suliman Alboloi
Shuyun Lee
Vlasta Ng
Shao Wei Toh
Catherine Akunna Nzerem
Hyeyoung Choi
Namrataben Nikul Suthar
Fong Lai Guan
Ernest Koh
Seongcheol Moon
Arvindaraj Pillay So Thangaraj
Lai Sin Sam
Janine Monica Amacio Balmorez
Virginia Garcia Canarias
Ernesto Moral Batin JR
Julien Taud Acoking
Maria Gelica Agonoy Dumlao
Margaret Christine Villareal Gonzalez
Barry Cañete Gundayao
Rajinder Kaur
Micah Manalo Estuye
Giovanni Resuello Barrozo
Pauline Camille Paglinawan Wood
Franchezca Amanda Tolentino Culala
Alexander Kevin Soriano Bulatao
Rolando Quiming Felix JR
Irene Manzana Ortiz
El Christopher Oliveros Alvarado
Cris Angel Blanca Morales Roque
Mrizhal Manalo Mediana
Nino Amiel Gerasta Solatorio
Margaret Wairimu Ithibu
Christian Angelo Vileta Reyes
Tariku Bekele Meloro
Dong Kyu Lee
Ingrid Marie Escaño Yap
Kenneth Jievynn Sales Qua
Janine Pelayo Pardo
Divine Grazel Manligas Panuayan
Sabah Suliman
Dahee Jung
Macey Puri Guina
Karen Alyanna Catudio Monsanto
Nicole Tiffanie Sy Tan
Danica Rose Barona Ocampo
Olivia Mago Derpo
Reynald Soriano Calagui
Amel Mohammed Khair
Razan Farah
Jalil Nasiri
Iven Christ Licos Castromayor
Jemal Alemu Ibrahim
Cheryl Datinguinoo Suello
Maechelle Pingoy Rosales
Jennylynn Dotillos Bo
Jennyvie Dallego Ariola
Melaku Tamene Asfaw
Chia Ju Yang
Elinor Mae Cabana Villacarlos
Cecilia Reva Libranda Tabugara
Maria Nelma Bajinting Sarte
Jing Wen Zhang
Nissren Mohamed
Raezel Jonn Ligutan Soriano
Namrataben Nikul Suthar
Gassan Elhuseen
Moaaz Bin Jable Ali
Sara Hassaballah
Eiman Yousif
Mowda Elmleeh
Wafa Elhag
Cecile Chavez Reynoso
International Phlebotomy
Technician, PBT(ASCP
i
)
Eman Hassan
Eldeluz Trocio Bongcaron
Amani Hamid Elhaga
International Technologist
in Gynecologic Cytology,
CTg yn (A SC P
i
)
Yosra M Osman
Marwa Awad
International Technologist in
Hematology, H(ASCP
i
)
Muhammad Saboor
Namgeun Park
All articles submitted to this publication are for information purposes only and do not necessarily reect the opinion or position of the ASCP BOC.
ASCP BOC has recently switched to a new system of tracking certicants. If you are not listed on this page, for the aforementioned dates, please contact
[email protected] and we will make sure to include you in the next issue of the International Certication Report.
